华西口腔医学杂志 ›› 2024, Vol. 42 ›› Issue (2): 192-206.doi: 10.7518/hxkq.2024.2023280
李凯玉( ), 石丽娟, 刘林鑫, 王杰, 聂敏海(
), 石丽娟, 刘林鑫, 王杰, 聂敏海( ), 刘旭倩(
), 刘旭倩( )
)
收稿日期:2023-08-29
修回日期:2024-01-22
出版日期:2024-04-01
发布日期:2024-03-26
通讯作者:
聂敏海,刘旭倩
E-mail:17860751351@163.com;nieminhai@126.com;liuxuqiankokky@126.com
作者简介:李凯玉,硕士,E-mail:基金资助:
Li Kaiyu( ), Shi Lijuan, Liu Linxin, Wang Jie, Nie Minhai(
), Shi Lijuan, Liu Linxin, Wang Jie, Nie Minhai( ), Liu Xuqian(
), Liu Xuqian( )
)
Received:2023-08-29
Revised:2024-01-22
Online:2024-04-01
Published:2024-03-26
Contact:
Nie Minhai,Liu Xuqian
E-mail:17860751351@163.com;nieminhai@126.com;liuxuqiankokky@126.com
Supported by:摘要:
目的 研究口腔黏膜癌变进程中基于数据计算验证的固有免疫细胞和免疫检查点分子的表达趋势,并通过预测其交互作用,探索免疫治疗抑制口腔黏膜癌变进程的方法。 方法 1)利用癌症基因组图谱对口腔黏膜癌变进程中的免疫细胞和免疫检查点分子进行全面评分,筛选出干扰肿瘤细胞免疫逃逸的固有免疫细胞和免疫检查点分子;2)收集血常规资料,对口腔黏膜癌变进程中外周血免疫细胞进行统计学分析,筛选外周血中可能影响口腔黏膜癌变进程的免疫细胞;3)对口腔黏膜癌变进程各阶段中基于数据计算验证的固有免疫细胞和免疫检查点分子进行免疫组织化学染色;4)采用特殊染色鉴定口腔黏膜癌变进程各阶段中基于数据计算验证的固有免疫细胞;5)对口腔黏膜癌变进程中基于数据计算验证的固有免疫细胞和免疫检查点分子进行生存分析,验证固有免疫细胞和免疫检查点分子与口腔鳞状细胞癌预后间的关联。 结果 在口腔黏膜癌变进程中,单核细胞、中性粒细胞表达呈上升趋势;嗜酸性粒细胞表达呈升降单峰趋势;肥大细胞表达呈下降趋势;免疫检查点分子细胞毒性T淋巴细胞相关蛋白4(CTLA4)和细胞程序性死亡-配体1(PD-L1)的表达呈上升趋势。单核细胞、中性粒细胞和嗜酸性粒细胞表达趋势与CTLA4和PD-L1免疫检查点分子的表达趋势正相关;肥大细胞表达趋势与CTLA4和PD-L1免疫检查点分子的表达趋势负相关。单核细胞、中性粒细胞和嗜酸性粒细胞可能促进CTLA4和(或)PD-L1介导的肿瘤细胞免疫逃逸,加速口腔黏膜癌变进程;肥大细胞可能抑制CTLA4和(或)PD-L1介导的肿瘤细胞免疫逃逸,延缓口腔黏膜癌变进程。 结论 干扰固有免疫中特定免疫细胞可在一定程度上调控CTLA4和(或)PD-L1的表达,抑制肿瘤细胞免疫逃逸,延缓口腔黏膜癌变进程。
中图分类号:
李凯玉, 石丽娟, 刘林鑫, 王杰, 聂敏海, 刘旭倩. 口腔黏膜癌变进程中固有免疫细胞与免疫检查点分子表达趋势验证及交互作用预测[J]. 华西口腔医学杂志, 2024, 42(2): 192-206.
Li Kaiyu, Shi Lijuan, Liu Linxin, Wang Jie, Nie Minhai, Liu Xuqian. Verification of the expression trend and interaction prediction of innate immune cells and immune-checkpoint molecules in the process of oral mucosal carcinogenesis[J]. West China Journal of Stomatology, 2024, 42(2): 192-206.
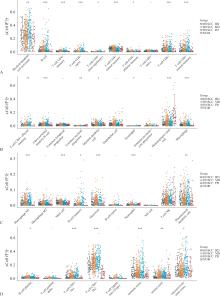
图 1
HNSCC组织和正常组织中免疫细胞评分的分布情况A:活化髓样树突状细胞、B淋巴细胞、CD4+记忆T淋巴细胞、CD4+幼稚T淋巴细胞、CD4+非调节性T细胞、CD4+中枢记忆T淋巴细胞、CD4+效应记忆T细胞、CD8+幼稚T淋巴细胞、CD8+T淋巴细胞、CD8+中枢记忆T淋巴细胞;B:CD8+效应T淋巴细胞、转换记忆B淋巴细胞、普通髓系祖细胞、淋巴样祖细胞、髓样树突状细胞、内皮细胞、嗜酸性粒细胞、粒-单核细胞祖细胞、造血干细胞、巨噬细胞;C:M1巨噬细胞、M2巨噬细胞、肥大细胞、记忆B淋巴细胞、单核细胞、幼稚B淋巴细胞、中性粒细胞、NK细胞、NK-T淋巴细胞、浆细胞样树突状细胞;D:B淋巴细胞基质、T淋巴细胞、CD4+辅助性T细胞1、CD4+辅助性T细胞2、调节性T细胞、免疫评分、基质评分、免疫微环境评分。横坐标代表免疫细胞浸润类型,纵坐标代表该免疫浸润评分在不同组的分布情况。*P<0.05,**P<0.01,***P<0.001,-差异无统计学意义。
表 5
各组间嗜酸性粒细胞数及其占比的比较情况
| 对比组次 | 嗜酸性粒细胞 | 嗜酸性粒细胞占比 | ||
|---|---|---|---|---|
| 数值/(×109/L) | 组间P值 | 数值/% | 组间P值 | |
| NOM与OSCC HD | 0.15与0.11 | 0.00 | 2.41与1.67 | 0.00 |
| NOM与OSCC MD | 0.15与0.11 | 0.03 | 2.41与1.81 | 0.01 |
| NOM与OSCC PD | 0.15与0.10 | 0.00 | 2.41与1.63 | 0.00 |
| OSCC HD与OSCC MD | 0.11与0.11 | 0.43 | 1.67与1.81 | 0.49 |
| OSCC HD与OSCC PD | 0.11与0.10 | 0.88 | 1.67与1.63 | 0.66 |
| OSCC MD与OSCC PD | 0.11与0.10 | 0.37 | 1.81与1.63 | 0.32 |
表 6
各组间嗜碱性粒细胞数及其占比的比较情况
| 对比组次 | 嗜碱性粒细胞 | 嗜碱性粒细胞占比 | ||
|---|---|---|---|---|
| 数值/(×109/L) | 组间P值 | 数值/% | 组间P值 | |
| NOM与OSCC HD | 0.02与0.03 | 0.14 | 0.39与0.44 | 0.32 |
| NOM与OSCC MD | 0.02与0.02 | 0.40 | 0.39与0.39 | 0.69 |
| NOM与OSCC PD | 0.02与0.03 | 0.20 | 0.39与0.42 | 0.58 |
| OSCC HD与OSCC MD | 0.03与0.02 | 0.64 | 0.44与0.39 | 0.56 |
| OSCC HD与OSCC PD | 0.03与0.03 | 0.93 | 0.44与0.42 | 0.63 |
| OSCC MD与OSCC PD | 0.02与0.03 | 0.71 | 0.39与0.42 | 1.00 |
表 7
各组间中性粒细胞数及其占比的比较情况
| 对比组次 | 中性粒细胞 | 中性粒细胞占比 | ||
|---|---|---|---|---|
| 数值/(×109/L) | 组间P值 | 数值/% | 组间P值 | |
| NOM与OSCC HD | 3.71与5.00 | 0.05 | 67.65与68.12 | 0.80 |
| NOM与OSCC MD | 3.71与4.50 | 0.03 | 67.65与66.83 | 0.70 |
| NOM与OSCC PD | 3.71与5.09 | 0.00 | 67.65与67.65 | 1.00 |
| OSCC HD与OSCC MD | 5.00与4.50 | 0.50 | 68.12与66.83 | 0.47 |
| OSCC HD与OSCC PD | 5.00与5.09 | 0.79 | 68.12与67.65 | 0.80 |
| OSCC MD与OSCC PD | 4.50与5.09 | 0.41 | 66.83与67.65 | 0.69 |
表 8
各组间淋巴细胞数及其占比的比较情况
| 对比组次 | 淋巴细胞 | 淋巴细胞占比 | ||
|---|---|---|---|---|
| 数值/(×109/L) | 组间P值 | 数值/% | 组间P值 | |
| NOM与OSCC HD | 1.87与1.53 | 0.00 | 30.74与23.64 | 0.00 |
| NOM与OSCC MD | 1.87与1.54 | 0.00 | 30.74与24.75 | 0.00 |
| NOM与OSCC PD | 1.87与1.60 | 0.02 | 30.74与24.45 | 0.00 |
| OSCC HD与OSCC MD | 1.53与1.54 | 0.89 | 23.64与24.75 | 0.48 |
| OSCC HD与OSCC PD | 1.53与1.60 | 0.47 | 23.64与24.45 | 0.61 |
| OSCC MD与OSCC PD | 1.54与1.60 | 0.79 | 24.75与24.45 | 0.87 |
| 1 | 王海青, 向婉婷, 卢俊米. PD-L1在口腔鳞状细胞癌中表达的临床意义[J]. 中国卫生标准管理, 2023, 14(6): 83-87. |
| Wang HQ, Xiang WT, Lu JM. Clinical significance of PD-L1 expression in oral squamous cell carcinoma[J]. China Health Stand Manag, 2023, 14(6): 83-87. | |
| 2 | Koike K, Dehari H, Ogi K, et al. Prognostic value of FoxP3 and CTLA-4 expression in patients with oral squamous cell carcinoma[J]. PLoS One, 2020, 15(8): e-0237465. |
| 3 | Wu T, Tang C, Tao R, et al. PD-L1-mediated immunosuppression in oral squamous cell carcinoma: relationship with macrophage infiltration and epithelial to mesenchymal transition markers[J]. Front Immunol, 2021, 12: 693881. |
| 4 | Sudo S, Kajiya H, Okano S, et al. Cisplatin-induced programmed cell death ligand-2 expression is associated with metastasis ability in oral squamous cell carcinoma[J]. Cancer Sci, 2020, 111(4): 1113-1123. |
| 5 | Yuan Y, Jiao P, Wang Z, et al. Endoplasmic reticulum stress promotes the release of exosomal PD-L1 from head and neck cancer cells and facilitates M2 macropha-ge polarization[J]. Cell Commun Signal, 2022, 20(1): 12. |
| 6 | Weber M, Lutz R, Olmos M, et al. Beyond PD-L1-identification of further potential therapeutic targets in oral cancer[J]. Cancers (Basel), 2022, 14(7): 1812. |
| 7 | Yu GT, Bu LL, Zhao YY, et al. CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma[J]. Oncoimmunology, 2016, 5(6): e1151594. |
| 8 | Starzyńska A, Sejda A, Adamski Ł, et al. The B7 family molecules in oral squamous cell carcinoma: a systematic review. PartⅠ: B7-H1 (PD-L1) and B7-DC (PD-L2)[J]. Postepy Dermatol Alergol, 2022, 39(2): 265-274. |
| 9 | Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer[J]. Cancer Res, 2013, 73(1): 128-138. |
| 10 | Zgodzinski W, Grywalska E, Zinkiewicz K, et al. Peripheral blood T lymphocytes are downregulated by the PD-1/PD-L1 axis in advanced gastric cancer[J]. Arch Med Sci, 2019, 15(3): 774-783. |
| 11 | Quan J, Morrison NA, Johnson NW, et al. MCP-1 as a potential target to inhibit the bone invasion by oral squamous cell carcinoma[J]. J Cell Biochem, 2014, 115(10): 1787-1798. |
| 12 | Moradi Tabriz H, Obohat M, Vahedifard F, et al. Survey of mast cell density in transitional cell carcinoma[J]. I-ran J Pathol, 2021, 16(2): 119-127. |
| 13 | Hadjigol S, Shah BA, O’Brien-Simpson NM. The ‘Danse Macabre’-neutrophils the interactive partner affec-ting oral cancer outcomes[J]. Front Immunol, 2022, 13: 894021. |
| 14 | Siddiqui S, Jaiswal R, Hashmi GS. Quantitative analysis of tumor-associated tissue eosinophils and tumor-associated blood eosinophils in oral squamous cell carcinoma[J]. J Oral Maxillofac Pathol, 2020, 24(1): 131-137. |
| 15 | Sharma HD, Mahadesh J, Monalisa W, et al. Quantitative assessment of tumor-associated tissue eosinophilia and nuclear organizing region activity to validate the significance of the pattern of invasion in oral squamous cell carcinoma: a retrospective study[J]. J Oral Maxillofac Pathol, 2021, 25(2): 258-265. |
| 16 | 李腾艳, 聂敏海, 陈潇, 等. 白细胞表达趋势在监测癌前病损和OSCC早期诊断中的价值研究[J]. 实用口腔医学杂志, 2020, 36(5): 753-757. |
| Li TY, Nie MH, Chen X, et al. The value of leukocyte expression trend in monitoring precancerous lesion and early diagnosis of OSCC[J]. J Pract Stomatol, 2020, 36(5): 753-757. | |
| 17 | 高岩. 口腔组织病理学[M]. 北京: 人民卫生出版社, 2020: 81-90, 181-182, 315-319. |
| Gao Y. Oral histology and pathology[M]. Beijing: People’s Medical Publishing House, 2020: 81-90, 181-182, 315-319. | |
| 18 | Marin-Acevedo JA, Kimbrough EO, Lou Y. Next gene-ration of immune checkpoint inhibitors and beyond[J]. J Hematol Oncol, 2021, 14(1): 45. |
| 19 | Shi L, Yang Y, Li M, et al. LncRNA IFITM4P promotes immune escape by up-regulating PD-L1 via dual mechanism in oral carcinogenesis[J]. Mol Ther, 2022, 30(4): 1564-1577. |
| 20 | Phulari RGS, Rathore RS, Shah AK, et al. Neutrophil: lymphocyte ratio and oral squamous cell carcinoma: a preliminary study[J]. J Oral Maxillofac Pathol, 2019, 23(1): 78-81. |
| 21 | Grimm M, Rieth J, Hoefert S, et al. Standardized pretreatment inflammatory laboratory markers and calculated ratios in patients with oral squamous cell carcinoma[J]. Eur Arch Otorhinolaryngol, 2016, 273(10): 3371-3384. |
| 22 | Teófilo CR, Ferreira Junior AEC, Batista AC, et al. Mast cells and blood vessels profile in oral carcinogenesis: an immunohistochemistry study[J]. Asian Pac J Cancer Prev, 2020, 21(4): 1097-1102. |
| 23 | Ansari FM, Asif M, Kiani MN, et al. Evaluation of mast cell density using CD117 antibody and microvessel density using CD34 antibody in different grades of oral squa-mous cell carcinoma[J]. Asian Pac J Cancer Prev, 2020, 21(12): 3533-3538. |
| 24 | Narayan KV, Sonia G, Shrestha P, et al. A comparative study of mast cells count in different histological grades of oral squamous cell carcinoma by using toluidine blue stain[J]. Cureus, 2020, 12(9): e10626. |
| 25 | Ingaleshwar PS, Pandit S, Desai D, et al. Immunohistochemical analysis of angiogenesis by CD34 and mast cells by toluidine blue in different grades of oral squamous cell carcinoma[J]. J Oral Maxillofac Pathol, 2016, 20(3): 467-473. |
| 26 | Lien MY, Chang AC, Tsai HC, et al. Monocyte chemoattractant protein 1 promotes VEGF-A expression in OSCC by activating ILK and MEK1/2 signaling and downregulating miR-29c[J]. Front Oncol, 2020, 10: 592415. |
| 27 | Gao L, Wang FQ, Li HM, et al. CCL2/EGF positive feedback loop between cancer cells and macrophages promotes cell migration and invasion in head and neck squamous cell carcinoma[J]. Oncotarget, 2016, 7(52): 87037-87051. |
| 28 | Debta P, Debta FM, Chaudhary M, et al. Evaluation of myeloid cells (tumor-associated tissue eosinophils and mast cells) infiltration in different grades of oral squamous cell carcinoma[J]. Indian J Med Paediatr Oncol, 2016, 37(3): 158-167. |
| 29 | Sahni P, Patel A, Md S, et al. Tumor associated tissue eosinophilia in oral squamous cell carcinoma: a histo-che-mical analysis[J]. Malays J Med Sci, 2015, 22(6): 21-25. |
| 30 | Goertzen C, Mahdi H, Laliberte C, et al. Oral inflammation promotes oral squamous cell carcinoma invasion[J]. Oncotarget, 2018, 9(49): 29047-29063. |
| 31 | Oliveira-Costa JP, de Carvalho AF, da Silveira da GG, et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells[J]. Oncotarget, 2015, 6(25): 20902-20920. |
| 32 | Hirai M, Kitahara H, Kobayashi Y, et al. Regulation of PD-L1 expression in a high-grade invasive human oral squamous cell carcinoma microenvironment[J]. Int J Oncol, 2017, 50(1): 41-48. |
| 33 | Kämmerer PW, Toyoshima T, Schöder F, et al. Association of T-cell regulatory gene polymorphisms with oral squamous cell carcinoma[J]. Oral Oncol, 2010, 46(7): 543-548. |
| 34 | Weber M, Wehrhan F, Baran C, et al. Prognostic significance of PD-L2 expression in patients with oral squamous cell carcinoma—A comparison to the PD-L1 expression profile[J]. Cancer Med, 2019, 8(3): 1124-1134. |
| 35 | Jiang M, Li B. STAT3 and its targeting inhibitors in oral squamous cell carcinoma[J]. Cells, 2022, 11(19): 3131. |
| 36 | Shrestha A, Keshwar S, Raut T. Evaluation of mast cells in oral potentially malignant disorders and oral squamous cell carcinoma[J]. Int J Dent, 2021, 2021: 5609563. |
| 37 | Wu MH, Hong HC, Hong TM, et al. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression[J]. Clin Cancer Res, 2011, 17(6): 1306-1316. |
| 38 | Verza FA, Valente VB, Oliveira LK, et al. Social isolation stress facilitates chemically induced oral carcinogenesis[J]. PLoS One, 2021, 16(1): e0245190. |
| 39 | Yellapurkar S, Natarajan S, Boaz K, et al. Tumour-associated tissue eosinophilia in oral squamous cell carcinoma—A boon or a bane[J]. J Clin Diagn Res, 2016, 10(4): ZC65-ZC68. |
| 40 | 陈晓琳, 杨于权, 侯照远, 等. 肿瘤相关中性粒细胞在肿瘤免疫治疗中的研究进展[J]. 中国免疫学杂志, 2023, 39(7): 1519-1524. |
| Chen XL, Yang YQ, Hou ZY, et al. Role of tumor-associated neutrophils in tumor immunotherapy[J]. Chin J Immunol, 2023, 39(7): 1519-1524. | |
| 41 | Wu CF, Hung TT, Su YC, et al. Endoplasmic reticulum stress of oral squamous cell carcinoma induces immunosuppression of neutrophils[J]. Front Oncol, 2022, 12: 818192. |
| 42 | Hu X, Xiang F, Feng Y, et al. Neutrophils promote tumor progression in oral squamous cell carcinoma by regulating EMT and JAK2/STAT3 signaling through che-merin[J]. Front Oncol, 2022, 12: 812044. |
| 43 | Dong Y, Wang Z, Mao F, et al. PD-1 blockade prevents the progression of oral carcinogenesis[J]. Carcinogenesis, 2021, 42(6): 891-902. |
| 44 | 陈志红, 吴亚东. 程序性死亡受体-1及其配体在口腔鳞状细胞癌中的研究进展[J]. 华西口腔医学杂志, 2020, 38(4): 449-453. |
| Chen ZH, Wu YD. Development of programmed death receptor-1 and programmed death receptor-1 ligand in o-ral squamous cell carcinoma[J]. West China J Stomatol, 2020, 38(4): 449-453. | |
| 45 | Suárez-Sánchez FJ, Lequerica-Fernández P, Suárez-Canto J, et al. Macrophages in oral carcinomas: relationship with cancer stem cell markers and PD-L1 expression[J]. Cancers (Basel), 2020, 12(7): 1764. |
| 46 | Kai K, Moriyama M, Haque ASMR, et al. Oral squamous cell carcinoma contributes to differentiation of mo-nocyte-derived tumor-associated macrophages via PAI-1 and IL-8 production[J]. Int J Mol Sci, 2021, 22(17): 9475. |
| [1] | 刘磊, 向中正, 李一, 郭伟, 杨凯, 王军, 孙志军, 任国欣, 张建国, 孙沫逸, 冉伟, 黄桂林, 唐瞻贵, 李龙江. 头颈部鳞癌免疫检查点抑制剂治疗专家共识[J]. 华西口腔医学杂志, 2022, 40(6): 619-628. |
| [2] | 郭伟. 晚期头颈恶性肿瘤程序性细胞死亡蛋白1免疫治疗的临床研究述评[J]. 华西口腔医学杂志, 2020, 38(5): 489-494. |
| [3] | 郅克谦 徐燕 任文豪 高岭 赵璐 杨勇 张引成. 不同方法制备抗原致敏的树突状细胞介导T淋巴细胞对Tca8113细胞杀伤作用的影响[J]. 华西口腔医学杂志, 2010, 28(02): 195-198. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||